|
THE AGES OF GAIA: A BIOGRAPHY OF OUR LIVING EARTH |
|||||||||
|
4: The Archean
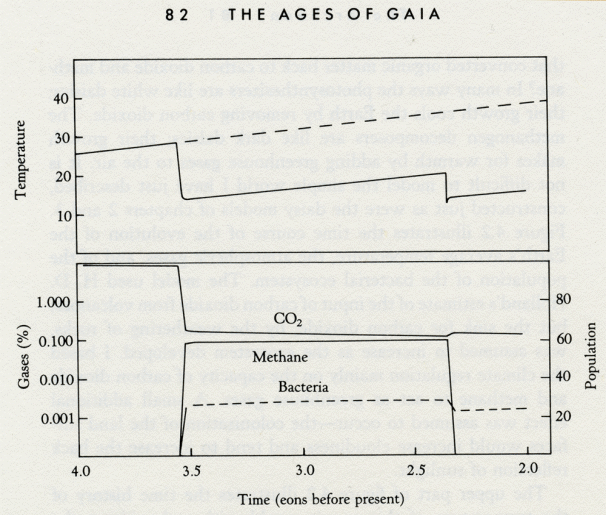
Life began a long time ago. The date of the event is not known, but it was at least three thousand six hundred million years before we were born. Numbers as large as this are anesthetic and paralyze the imagination. A different scale of reckoning time is needed to reach back to those bacteria, our ultimate grandparents. In science, the usual way of taming outrageous numbers is to express them as powers of ten. Make every step ten times larger or smaller than the one before. In his book Timescale: An Atlas of the Fourth Dimension, Nigel Calder illustrates the Earth's history in this way. He reminds us how easily this logarithmic sense of time can prevent us from recognizing how long life has occupied the Earth; to say that life began 3.6 x 109 years ago does not help. On a linear scale of measure, the origin of life was about a thousand times more remote than the origin of humans. In this book, I shall use a scale of eons, which represent a thousand million years. Life started at least 3.6 eons ago, during the period geologists call the Archean, the period that runs from the Earth's assembly 4.5 eons ago to 2.5 eons ago when oxygen first dominated the chemistry of the atmosphere. Gaia is as old as life; indeed, if the Big Bang that started the Universe was 15 eons back from now, she is a quarter as old as time itself. She is so old that her birth was in the region of time where ignorance is an ocean and the territory of knowledge is limited to small islands, whose possession gives a spurious sense of certainty. In this chapter I invite you to join with me in speculating about the infant Gaia and the problems she faced in taking on her inheritance, the Earth. When we look at the Archean period in the light of Gaia theory, we see a planet radically different from that depicted by the conventional wisdom of present-day science. It is a planet where life does not just adapt to the Earth it finds itself upon, but also adapts the Earth to make it and keep it a home. The best way to illustrate the powerful presence of Gaia is to consider what the Earth would be like without life. It will be argued that the present-day Earth could be an arid place like Mars or Venus had life not appeared upon it. We cannot make such a comparison for the Archean because we know so little about the Earth then. What we must do, therefore, is make a best guess about the condition of the Earth before life, and then consider the changes there would be when life took charge. By asking what the Earth was like before life began, we are in a way hanging up a neutral back cloth before which can be clearly seen the colorful changes made by life. The trouble with doing this is that the back cloth is so old as to have all but moldered away. Looking back in time is like using a telescope to view the limits of the Universe. We can see faintly luminous objects. Astronomers make a convincing case that the distance of these objects is so great that the light now seen started its journey to the Earth 3.8 eons ago. This is close to the time geologists believe the first bacterial cells came into existence. They are probably correct, but the only certainty about such remote times and places comes from the great second law of thermodynamics. Enigmatically, it states that the beginning and the end of the Universe are unknowable. As time and distance lengthen, the once fair face of knowledge grows pockmarked with craters of ignorance. In the end, the features can no longer be recognized. Information theory teaches that, in the presence of a constant amount of noise, the power required to send a signal across a gulf of space and time increases exponentially with the distance to be traveled. In simpler words: as the distance or the time lengthens, vastly more power is needed to transmit the same message. The happenings on Earth a mere 5000 years ago are far from known with certainty. Just imagine how large a signal would be needed to transmit information about the beginning of the Universe 15 eons ago. This may be why the Big Bang theory that the Universe began by the explosion of a primeval particle is inevitable. Nothing short of the explosion of the Universe itself could send a signal from so long ago. All that now lingers is the faint rumble of the cosmic microwave background radiation. But all other theories of the origin are without evidence. There is a clever way to gather information about events as ancient as the start of life that avoids the otherwise universal tendency of messages to age and die. It comes from the nearly miraculous property of living matter to overcome the attenuating tendency of time. Not only has Gaia stayed alive from the beginning; she has also provided a noise-free channel of chemical messages about those ancient times. If you stand on a hilltop and shout, you will not be heard more than a mile away. If you use a loud-speaker, you might be able to send a message 5 miles. Even exploding an H-bomb would make your point only for a few hundred miles. The alternative is to tell a friend who will take the message and pass it on by word of mouth. By this means, the message could travel without difficulty to the ends of the Earth. In a similar way, living organisms pass on the programs of the cell from one generation to the next. There is every reason to believe that we share with the first ancient bacteria a common chemistry, and that the natural restrictions on the existence of those ancient bacteria tells us what the environment of the early Earth was like. By transmitting coded messages in the genetic material of living cells, life acts as a repeater, with each generation restoring and renewing the message of the specifications of the chemistry of the early Earth. It is a much better channel of information than the record of the rocks. It is precise, but unfortunately it is inaccurate in the way that a message passed by word of mouth is precise and makes sense, but inevitably "mutates." There is the wartime joke that hides a truth: how the message passed by word of mouth, "Send reinforcements, we are going to advance" mutated into "Send three and four pence, we are going to a dance." If we wish to know life's origins from genetic information we need to be prepared to reconstruct the truth from errors of this kind. By contrast, most of the geological information about the early Earth came from another big bang. It had to be large to send a signal so far. It was the explosion of a star-sized nuclear bomb, a supernova. We tend to ignore that we oddities, who use combustion as a source of energy, inhabit a nuclear-powered Universe. The power plants, the stars, run for billions of years with utmost reliability. But just as the most dependable systems we design can still have the occasional accident, so some kinds of stars occasionally explode. Fortunately for us, one of them did and gave us the start we needed. Fortunately, also, our Sun is not of the exploding kind; it is neither big enough nor old enough. How can we be so sure that the Earth's origin was connected with the explosion of a supernova? We are sure because, even today, the Earth is radioactive, and also because the Earth is made of elements like iron and silicon and oxygen that cannot be made in the normal processes of stellar evolution. In the Sun and similar stars, hydrogen is fused to generate helium, and the reaction liberates the vast outpouring of heat that keeps us warm even 100 million miles away. But no ordinary fusion process can make elements such as iron, nor those such as uranium, which are heavier. It takes energy to make such elements. Powering a star by fusing iron to make uranium is like trying to burn ice in a furnace. This is not the place for fine details of element synthesis in exploding stars, except to say that in one kind of explosion the key part of the event is the gravitational collapse of the star. The innermost regions support the fantastic pressure of all the mass of the star trying to fall in. In their active life, the heat generated by nuclear reactions at the center of the star sustains a pressure high enough to balance the inward force of gravitation. It is just like a space rocket at the moment of takeoff; the weight of the vehicle is supported by the blast of flame. But the outer layers of the star cannot escape the pull of gravitation and, when the fuel runs out, it collapses. It is then that the heavy elements are synthesized. Some proportion of them is violently ejected as the outer and still unburnt layers of the star explode. We still do not know how the Solar System and the Earth came together as a result of that supernova; nor how its radioactive debris became so large a part of our planet. But radioactivity is a marvelously accurate clock, and has precisely ticked away the time since that explosion 4.55 eons ago. We are so used to thinking of radioactivity as artificial that we easily ignore the fact that we ourselves are naturally radioactive. Every minute, in each one of us, a few million potassium atoms undergo radioactive decay. The energy that powers these minuscule explosive atomic events has been locked up in potassium atoms ever since that stellar explosion long ago. The element potassium is radioactive but it is also essential for life. If it were removed and replaced by the very similar element, sodium, we should die instantly. Potassium, like uranium and thorium and radium, is a long-lived radioactive nuclear waste of the supernova bomb. When potassium atoms decay, they are transmuted to form atoms of calcium and of the noble gas argon. The one percent of argon that goes to make up the atmosphere has, over the course of the Earth's history, mostly come from potassium in this way. In the rocks, the radioactive elements uranium and thorium are present at several parts per million. Their rate of decay is so slow that most of what was originally present still remains, except for the uranium isotope 235U, nearly all of which has decayed. It is the heat generated by the decay of these radioactive elements that keeps the Earth's interior hot and drives the movements of the crust. The evidence from the rocks suggests that life began between 0.6 and 1 eon after the Earth had come together as a recognizable planetary body. The evidence is a difference in the proportions of the atoms of the stable element carbon. Carbon atoms exist on Earth in three forms: the common form weighs 12 atomic units, but there is a proportion weighing 13 units and a small trace of radioactive carbon weighing 14 units. These different-weight atoms are called isotopes. The proportion of the 12 and 13 isotopes, in the carbon of rocks made in the absence of life, is recognizably different from the proportion in carbon from rocks that were once living matter; this is because the chemistry of living matter segregates the isotopes. By measuring the isotopic composition of ancient rocks it is possible to distinguish those that were made when life was present from those that formed before life began. The most certain pre-life rocks we have come not from the Earth but from the Moon or from meteorites. These are as old as 4.55 eons. The isotopic proportion of these dead-matter rocks is easily distinguished from those laid down on Earth 3.6 eons ago. The oldest sedimentary rocks on Earth so far found are 3.8 eons old, and they come from a place called Isua in Greenland. I recall the German geochemist Manfred Schidlowski describing these ancient rocks in a 1973 lecture, and speculating that the carbon atoms within them when they formed showed an isotopic distribution suggestive of the presence of life. The period before life has left no rocks from which we could reconstruct the details of the environment in which they formed; 4 eons or more of weathering and grinding has erased the record. It is likely to have been a time of unimaginable violence, with small planets left over from the condensation of the Solar System still crashing in. (The impact of a planetesimal a mere 6 miles in diameter can leave a crater 200 miles across, and splash molten rock and gas far out into space.) It left an Earth as cratered as the Moon. It was a period well named the Hadean. The chemistry and physics of the period just before life began can only be surmised, and it will be interesting to watch as speculations blossom about the amazing and turbulent history of the Earth's beginnings. You can see, however, the difficulty in weaving the neutral back cloth referred to earlier. Therefore we will have to make the best we can of the information available, starting with the atmosphere. The atmosphere is the face of the planet, and it tells, just as do our faces, its state of health and even if it is alive or dead. As we saw in chapter 1, planetary life is obliged to use any mobile media -- that is, the air or the oceans -- as conveyors of raw materials and as conduits for waste products. Such a use of these fluid media leads to profound changes in their chemical composition and to their departure from the near-equilibrium steady state characteristic of a nonliving planet. Dian Hitchcock and I used the absence of such changes in the atmosphere of Mars and Venus as evidence for the absence of life long before the Viking and Venera landers looked for and failed to find it. These dead planets are visually as well as chemically a neutral background against which the living planet Earth shines like a dappled sapphire. There are many reasons why the atmosphere is so much more revealing about life than are the ocean or the crustal rocks. It is the region of rapid chemical change under the influence of sunlight; no mixture of gases capable of chemical reaction can long remain unchanged in the atmosphere. If we find a combustible gas like methane present with oxygen in a sunlit atmosphere, we know for certain that something is constantly making them both. No such conclusion could be drawn about air in a sealed underground cave. It is the sunlight that constantly keeps ignited all possible chemical combustions. Then the atmosphere has the smallest mass of all the compartments that life encounters; apart from the small concentration of rare gases like argon and helium, all other gases of the air have recently existed as part of the solids and liquids of living cells. The atmosphere also has an immediate effect on the climate and chemical state of the Earth, features of fundamental importance to life. A similar exchange takes place between life and the oceans and the rocks, but it is much slower in pace and the cycles of life are diluted by materials used long ago but now discarded. The Earth, just before it became the habitat of life, then, must have been a dead planet whose atmosphere was near to equilibrium. At this time just before life, before Gaia, the atmosphere would have been in what scientists call the "abiological steady state." This wordy phrase is to distinguish the real planet -- which has hurricanes and tornadoes, volcanoes and whirlpools -- from the fiction of the utter stillness of an equilibrium planet. The early Earth is thought to have had on its surface the chemical components from which life assembled, chemical compounds that are called "organic" -- such as amino acids, the subunits of protein; nucleosides, the subunits of the molecules of our cells that carry their genetic information; sugars, the subunits of polysaccharides; and many other essential parts waiting for the final act of assembly. It is important to recognize that these chemicals, although we regard them as characteristic of life, are also the products of the abiological steady state. The mere presence of such compounds on an oxygen-free planet is not by itself evidence for life. It is evidence only of the possibility of its formation. Not only was the Earth's chemistry just right for life to start, the climate also must have been favorable. Some ancient rocks show evidence of having been formed by the sedimentation of particles. Their layered structure suggests an origin in a shallow lake or sea and, therefore, of the presence of free water. The existence of life and pre-life chemicals requires a temperature range between 0 and 50°C. The Earth could not have been frozen, nor could it have been hot enough for the seas to boil. In an important paper in 1979, three atmospheric chemists and climatologists, T. Owen, R. D. Cess, and V. Ramanathan, reported calculations to determine the average temperature of the Earth at the time life began. They used the general consensus of astrophysicists, that stars grow hotter as they age, and supposed that the output of heat from the Sun was 25 percent less than it is now. They took values for the approximate amount of carbon dioxide gas that had escaped (or outgassed) from the Earth's interior. From this, they were able to calculate that the mean surface temperature of the Earth was 23°C; typical of the tropics today. Their calculations required the presence of 200 to 1000 times as much carbon dioxide in the air as there is now. Much would depend on the quantity of nitrogen present. If then, as now, nitrogen was the principal atmospheric gas, then the lower pressure of carbon dioxide would have sufficed. Also important, according to my friend the climatologist Ann Henderson-Sellers, would have been the distribution of water as oceans, snow, ice, clouds, and water vapor. Not surprisingly there is still debate about the climate on the occasion of life's start. Calculations by the climatologist R. J. Dickinson in 1987 suggest it may have been a few degrees cooler, in other words just about the same as now. The idea was that the lack of warmth of a cooler Sun could have been offset by a blanket of "greenhouse" gas. Gases with more than two atoms in their molecules have the interesting property of absorbing the radiant warmth, the infrared radiation, that escapes from the Earth's surface. These gases, which include carbon dioxide, water vapor, and ammonia, are transparent to the visible and the almost visible infrared radiation. These are the parts of the Sun's spectrum that carry most of its energy; radiant heat in this form will penetrate the air and warm the surface. The same gases are opaque to the longer wavelength infrared that radiates from the Earth's surface and lower atmosphere. The trapping of the warmth, which otherwise would escape to space, is the "greenhouse effect"; so called because it is like, although not the same as, the warming effect of the glass panes of a greenhouse. The first proposal that a gaseous greenhouse warmed the Earth was made by a distinguished Swedish chemist, Svante Arrhenius, in the last century. H. D. Holland, in The Chemical Evolution of the Atmosphere and the Oceans, gives a clear and readable statement of the probable state of the Earth just before Gaia awoke. In summary, he proposes an Earth with an atmosphere rich in carbon dioxide, with nitrogen present but bereft of oxygen, and with traces of gases such as hydrogen sulfide and hydrogen present. The oceans were laden with iron and other elements and compounds that can only exist in solution in the absence of oxygen. Among these could have been reduced compounds of sulfur and nitrogen. The presence of these gases and substances is important, because they are reducing agents -- they readily react with, and so remove, oxygen. Such an Earth would have a vast capacity to absorb oxygen and prevent its appearance in the free state. This proposal seems so reasonable that I shall take it as if it were a fact and use it as a key to understanding the evolution of the Archean period of the Earth's history. One other condition of the nascent Gaia is that three times as much internal heat was produced as now. This was because the Earth was more radioactive; less time had elapsed since the supernova that made it, and the fallout was still hot. It would be wrong, though, to think that this internal heat had an appreciable effect on the surface temperature of the Earth. The heat flux from below was trivial compared with that received from the Sun. The principal effect of greater production of internal heat would have been more vigorous volcanism, a higher output of gas to the air, and a more rapid reaction of volcanic rocks with the ocean waters. One of these reactions, that between the ferrous iron of basalt rock and water, can produce hydrogen gas. The continuous production of hydrogen would have had two important consequences. First, the maintenance of an oxygen-free atmosphere and surface favorable for life chemicals to accumulate. Second, the loss of hydrogen to space. The Earth's gravitational field is not strong enough to hold down the light atoms of hydrogen. If hydrogen escape had continued, we might have lost much of the oceans or even arrived at the arid state of Mars and Venus. (Such an escape cannot take place now because hydrogen would react biochemically in the oceans and with the abundant oxygen in the atmosphere to form water. Although it carries two hydrogen atoms, water is too heavy a molecule to escape directly into space. Another restraint on the direct loss of water from Earth is its tendency to freeze out and fall back as ice crystals from frigid regions of the air.) That, then, was the Earth before life. We can accept as reasonable the view that life started from the molecular chemical equivalent of eddies and whirlpools. The power that drove them was the flux of energy from the Sun and also the free energy of a hot young Earth. Prigogine and Eigen have plausibly formulated the physical mechanisms by which chemicals and cyclical reactions come together as dissipative structures of protolife. The stepwise evolution from protolife to the first living cell by a process of natural selection does not seem to me so difficult an intellectual pill to swallow. It would be interesting to know if protolife was tightly coupled to its environment and had the capacity to regulate. Two geochemists, A. G. Cairns-Smith and Leila Coyne, have alternatively suggested that the solids of the environment played a crucial part in life's origin. To my mind their ideas help to crystallize the supersaturated arguments, even though their details are disputed. The problem with dissipative structures of the fluid state is that they dissipate too soon. If they are to evolve to more permanent structures, something solid is needed to serve as an anchor or to house them. Again, the mental image of a wind instrument like a flute is helpful in this otherwise confusing topic. Just blowing makes a hiss of unruly dissipating eddies. But when the flutist blows across the port hole of the flute, the eddies are caught and tamed within the solid bounds of its hollow resonant tube to emerge as coherent musical notes. In their evolution, living organisms, too, seem to have used the security of the solid state of matter to store and pass on to their descendants the message of existence. The special solid state of the aperiodic crystals of DNA store the programs of the cell, and give organisms a span far beyond that of a dissipating eddy or a chemical cycle. The first living cells may have used as food the abundant organic chemicals lying around; also the dead bodies of the less successful competitors and the bodies of the successful ones that died of natural causes. These supplies of raw material and energy may soon have become scarce, and at some early time organisms discovered how to tap the abundant and inexhaustible energy of sunlight to make their own food. It is thought that the first of the photosynthesizers used the less demanding photochemical dissociation of hydrogen sulfide. Soon the real prize, how to use light energy to break the strong bonds binding oxygen to hydrogen and carbon, was won. Bacteria now called cyanobacteria, because of their blue-green color, did just this and are the predecessors of all green plants that now exist. There was a complete planetary system in the Archean. At the surface -- in the sunlight -- there were the primary producers, cyanobacteria (ancestors of those shown in figure 4.1), that used solar energy to make organic compounds and replicate themselves. They also would have made oxygen, but the abundance of reactive inorganic chemicals in their environment would have kept this gas close to the site of its production. Also present in the early ecosystem were the methanogens that gained material and some energy by rearranging the molecular products of the producers. The presence of these "scavenger" organisms would have assured the continuous disposal of the products and corpses of the photosynthesizers and the return to the environment of the essential element carbon as methane and carbon dioxide. They could not, as we and animals do, eat the cyanobacteria and use the food they had synthesized; to do this they would have needed oxygen. I suspect that the origin of Gaia was separate from the origin of life. Gaia did not awaken until bacteria had already colonized most of the planet. Once awake, planetary life would assiduously and incessantly resist changes that might be adverse and act so to keep the planet fit for life. Sparse life hanging on in oases could never have the power to regulate or oppose the unfavorable changes that are inevitable on a lifeless planet. Sparse life would only be found at the birth or death of a Gaian system. 4.1 Photomicrographs of cyanobacteria. These are the organisms that first used the energy of sunlight to produce organic materials and oxygen. They have been, both in the free state, and as endosymbionts, the primary producers from the beginning of the Archean until now. (Photographs courtesy of Michael Enzien.) The successful evolution of the photosynthesizers could have led to the first environmental crisis on Earth, and I like to think the first evidence of Gaia's awakening. In gaining their energy, the photosynthesizers would have used the carbon dioxide of the air and the oceans as their source of carbon. Just as we have a carbon dioxide problem now, so might they. We are beginning to realize that the benefits of burning fossil fuel as a source of energy are offset by the dangers inherent in the accumulation of carbon dioxide; it could lead to overheating. The danger faced by the photosynthesizers was the reverse. The cyanobacteria used the carbon dioxide as food. They were eating the blanket that kept the Earth warm. There was at that time a vigorous input of carbon dioxide from volcanoes, but the potential capacity of the bacterial sink could have far exceeded this source. If there had been only photosynthesizers, their abundant bloom over the oceans and on the surface could have reduced the carbon dioxide in a few million years to dangerously low levels. Long before the cyanobacteria ran out of carbon dioxide to eat, the Earth would have cooled to a frozen state and life could have persisted only where heat from below could melt the ice, or moved into a cycle of freezing and thawing as carbon dioxide from volcanoes accumulated and was then removed again. I think that neither of these calamities ever happened. The persistent presence of sedimentary rocks from 3.8 eons ago until now suggests that liquid water has always been present and the Earth has never been entirely frozen. What I would like to propose is a dynamic interaction between the early photosynthesizers, the organisms that processed their products, and the planetary environment. From this there evolved a stable self-regulating system, a system that kept the Earth's temperature constant and comfortable for life. Before venturing further into this imaginary reconstruction of life with Gaia in the Archean, I must emphasize that it will be no more than a flight of fancy. Solid evidence from the early Archean is scarce, and many different models can be made of it. The eminent geologist, Robert Garrels, often reminds me that in his model of the early Earth, the carbon dioxide remained abundant (about 20 percent by volume) and the Earth was hot (40°C or higher). The point of my model is not to argue for one or other global Archean ecosystem, but rather to illustrate how Gaia theory provides a different set of rules for planet models. The possible climatologies and geologies of a living planet are wholly different from those of a dead planet bearing life as a mere passenger. Having said this, let us continue with our "let's pretend." In the Archean, photosynthesizers used carbon dioxide and converted it to organic matter and oxygen or its equivalent; just as plants do today. The oxygen would have been mopped up immediately by the ubiquitous oxidizable matter of the environment; the iron and sulfur in the oceans. There was no significant population of oxidizing consumers grazing the photosynthesizers and returning carbon to the environment as carbon dioxide. There was, except in juxtaposition with the producers, no oxygen for consumers to breathe. Instead, there were the methanogens, scavengers and descendants of the original decomposers of organic chemicals. These early bacteria, capable of existence only in the absence of oxygen, lived by decomposing organic matter and converting the carbon in it to carbon dioxide and methane which they return to the air. They served in the Archean, like the consumers of today, to return to the air almost as much carbon as had been removed by the photosynthesizers. But what of the methane? Methane is a greenhouse gas like carbon dioxide, but it is much less stable in the atmosphere; it decomposes in solar ultraviolet light and reacts with hydroxyl radicals -- small molecules, with one atom each of hydrogen and oxygen, that are amazingly reactive and scavenge from the air all but the most stable molecules. It is reasonable to suppose that, in the Archean, this photochemical reaction zone would have been high in the atmosphere but at a level where the air was still dense enough to absorb ultraviolet. When ultraviolet breaks down methane, the products combine and recombine with other molecules to form a suite of complex organic chemicals. Suspended high in the stratosphere, these products could include droplets and particles; an upper-atmospheric smog. Such a layer could have profoundly changed the Archean environment. In its presence, the ultraviolet and visible radiation from the Sun would have been absorbed, and the region where the absorption occurred would have grown warmer. The presence of this warm layer in the atmosphere placed an "inversion" lid on the lower atmosphere, and would have reversed the normal tendency for a fall in temperature as one ascends from the surface. In other words, methane smog would have been the Archean equivalent of the ozone layer and would have acted, just as ozone does, both to stabilize the existence of the stratosphere and to filter out ultraviolet radiation. The existence of a lid, the "tropopause," above the lower atmosphere would have reduced the flux of methane to the regions where it was destroyed by ultraviolet; just as the foul air is trapped beneath the inversion layer of this century's air-pollution smogs. By this means, the methane concentration could have built up sufficiently to be useful as a greenhouse gas; also, its reaction products in the stratosphere, including water vapor, would have served in the same way. The screening out of ultra-violet by the smog layer would have protected other unstable gases such as ammonia and hydrogen sulfide and allowed them to accumulate to some extent in the lower atmosphere. Ultraviolet normally decomposes hydrogen sulfide and other similar gases, both directly and by other photochemical reactions that produce the hydroxyl radicals. It is conceivable that the lower atmosphere, shielded by the methane smog, contained some free oxygen coexisting with an excess of methane; just as there is free methane in small quantities coexisting in an excess of oxygen in the air we breathe now. This would be even more probable if the photosynthesizers existed in self-contained communities at the surface. Some of the oxygen they made would then diffuse into the air and persist for much longer than that released into the oxygen-hungry waters of the oceans. In a fully detailed model, we ought to include gases such as nitrous oxide, carbonyl sulfide, and methyl chloride; all are components of our present atmosphere. For this model, it is enough to bear in mind this possibility and the amazing and intricate series of reactions and consequences that could come from their presence. How stable would a planetary ecosystem be that was made up from photosynthesizers using carbon dioxide and decomposers that converted organic matter back to carbon dioxide and methane? In many ways the photosynthesizers are like white daisies; their growth cools the Earth by removing carbon dioxide. The methanogen decomposers are like dark daisies; their growth makes for warmth by adding greenhouse gases to the air. It is not difficult to model the simple world I have just described, constructed just as were the daisy models of chapters 2 and 3. Figure 4.2 illustrates the time course of the evolution of the Earth's average temperature, the atmospheric gases, and of the population of the bacterial ecosystem. The model used H. D. Holland's estimate of the input of carbon dioxide from volcanoes, but the sink for carbon dioxide, by the weathering of rocks, was assumed to increase as the ecosystem developed. I based the climate regulation mainly on the capacity of carbon dioxide and methane to act as greenhouse gases. A small additional effect was assumed to occur -- the colonization of the land surfaces would increase cloudiness and tend to increase the back reflection of sunlight. Time (eons before present) 4.2 Model of the Archean before and after life. The upper panel shows the climate with and without life and the lower panel the abundance of the atmospheric gases and bacterial population as the system evolved. The scale for the abundance of atmospheric gases is logarithmic; the scale for population is in arbitrary units. The upper part of figure 4.2 illustrates the time history of the temperature of this anoxic world with and without the presence of life. The dashed line is the expected temperature rise of a lifeless planet that has enough carbon dioxide to make up an atmospheric pressure of 100 millibars; about one-tenth of the present total atmospheric pressure. The bulk of the atmosphere was assumed to be nitrogen as it is now on Earth. The star was assumed to be 25 to 30 percent less luminous than the Sun is now, but to warm up as time passed in the same way as did the Sun. The solid line marks the temperature of the model world where photosynthesizers are coexisting with methanogens. Note the abrupt and sudden fall in temperature from around 28°C to 15°C after life starts. This is due to rapid decline in the abundance of the greenhouse gas, carbon dioxide, as the photosynthesizers use it to build their bodies. The fall does not continue until the planet freezes because the new greenhouse gas, methane, and some carbon dioxide are returned to the air by the methanogens. Once a steady state is established, throughout the Archean. The sudden fall in temperature at about 2.3 eons ago marks the end of the Archean in the model and the appearance of an excess of free oxygen in the air. This event would have led to a decline of methane gas to near its contemporary abundance, thereby removing its greenhouse effect. The model matches the Earth's ancient history. There is no evidence of unusual temperature change during the Archean, and there was a cold glacial period 2.3 eons ago that may have coincided with the appearance of atmospheric oxygen. The lower part of figure 4.2 shows how the total population of bacteria the model evolved. The start of life is seen to coincide with the fall of carbon dioxide and the rise of methane. The end of the Archean is marked by the disappearance of methane. This simple model, like Daisyworld, is robust and is not easily disturbed by changes in solar input, bacterial population, or the input of carbon dioxide from volcanic sources. It is sensitive to changes in the range or form of the relationship between the growth of the bacteria and the temperature of their environment. The model is based on the assumption that growth of the bacterial ecosystem ceased at freezing point, was maximum at 25°C, and ceased again at temperatures above 50°C. Like Daisyworld, there is an abrupt change of conditions when life starts. Living organisms grow rapidly until a steady state is reached where growth and decay are in balance. This rapid, almost explosive, tendency to expand to fill an environmental niche acts as an amplifier. The system moves rapidly in positive feedback to approach a balance. Soon stability is achieved and the planet runs on in comfortable homeostasis. The atmosphere of this new model of the Archean would be like a somewhat diluted version of the gas above a septic tank or a biogas generator -- smelly and toxic for us, but delightful for the denizens of those ancient times. The atmospheric abundance of both carbon dioxide and methane would range between 1.0 and 0.1 percent. It is interesting that H. D. Holland was doubtful about the continuation of an atmosphere with a high content of carbon dioxide for long into the Archean. The rates of rock weathering from the geological record are not consistent with the persistence of 10 percent or more carbon dioxide. The rapid removal of carbon dioxide by the bacterial ecosystems neatly removes this problem. It is worth noting that many kinds of bacteria, not just photosynthesizers, actively remove and use carbon dioxide and make chemical compounds from it.
According to the model, the atmosphere was wholly different in composition in the Archean after life began. Table 4.1 illustrates the mixing ratio of the principal atmospheric gases before and after life. It shows an increase in nitrogen abundance after life began: I speculated that, until then, some of the nitrogen was present as the ammonium ion (NH4)+ in the oceans. The sea was more acid from the excess of carbon dioxide, and was rich in ferrous iron. In these circumstances, the ferrous iron may well have sequestered a large proportion of the ammonium ion to make a stable iron-ammonia complex compound, in which form much of the element nitrogen would have existed. Both the fall in carbon dioxide and the use of nitrogen by life could have changed the balance in favor of nitrogen gas in the air. Although nitrogen has no greenhouse effect by itself, the increase in nitrogen would have doubled the atmospheric pressure and this would have increased the greenhouse effect of the carbon dioxide and methane gases. The reason for this is somewhat recondite, but is connected with an increase in the amount of infrared absorbed by the greenhouse gases when total atmospheric pressures are higher. It is important to note that there are other equally plausible models of the Archean. The conventional wisdom is expressed in Holland's book; it sees the pre-life environment continuing unchanged. Robert Garrels prefers to see the period as one where there were high temperatures sustained by high concentrations of carbon dioxide in the air. It is likely to be a long time before we are certain about the ancient history of the Earth. The purpose of this chapter, however, is not to make a firm statement on conditions during the Archean; it is to show how Gaia theory can be used to build from the meager evidence a different picture of those times. I like to imagine some alien chemist arriving in the Solar System long ago and viewing the Earth's pre-life atmosphere. The infrared spectrometer aboard the spacecraft would recognize a planet in the abiological steady state -- a planet not yet alive, but with the potential to bear life. On a second visit much later in the Archean, when life had taken charge, a similar analysis would show a degree of chemical disequilibrium impossible for a lifeless planet. Carbon dioxide, methane, hydrogen sulfide, and oxygen cannot coexist at the levels shown in table 4.1 in the presence of sunlight. Given the destructive effects of solar ultraviolet radiation on methane, oxygen, and hydrogen sulfide, the alien would know that there was a large, continuous source of these gases. No conceivable volcanic source could sustain such an atmosphere. The alien would conclude that the Earth was now alive. I often wonder what the Archean Earth would have looked like to us. I suspect that from an orbiting spacecraft we would not have seen the familiar blue-and-white sphere with glimpses of land and sea beneath the aerial canopy. More likely, the view would have been of a brownish-red, hazy planet; like Venus or Titan, too obscured to see the surface below. The sky that now we see as blue and clear results from an abundance of oxygen. Oxygen is the permanent bleach that clears and freshens the air. On a beach on the edge of an Archean continent, we would see waves breaking on smooth sand, and sloping dunes behind. It would be familiar except for the colors. The Sun high above would have an orange glow more like sunset. The sky would be a pinkish hue, and the sea, that great copyist, shades of brown. There would be neither shells nor tracks of moving things upon the sand. The breakers offshore would fall away at low tide, exposing reefs of the strange mushroom-shaped stromatolites formed by the calcium carbonate secreted by colonies of living cyanobacteria. Inland, behind the sand and shingle dunes, would be flat and stagnant water, with patches of matted green and black bacterial growth. Other than the wind and waves, the only sound would be the plop of methane bubbles bursting as they broke from containment in the mud. Beyond the lagoon and on the continental surface, the same scene would repeat wherever there were shallow depressions in which water could gather. On the drier land and on the hill sides, a thin varnish of microbial life would ceaselessly work at weathering the rocks, releasing nutrients and minerals into the flow of rain water, and continuously removing carbon dioxide from the air. This quiet landscape could have persisted throughout much of the Archean. But there would have been violent interruptions when planetesimals crashed in from space. There were at least ten of these collisions; each a catastrophe great enough to destroy more than half of all planetary life. They would have changed the physical and chemical environment enough to hazard the remainder of life for hundreds if not thousands of years to follow. It is a tribute to the strength of Gaia that our planetary home was restored so promptly and effectively after these events. Without life, the scene would have been much different. The ineluctable forces of chemical and physical evolution drive the small inner planets to an oxidized state through the loss of hydrogen. Venus must have had some water in the beginning. Estimates from the abundance of the unreactive noble gases suggests that, when the planets formed, Venus may have had at least a third as much water as the Earth. Where did it go? It seems most probable that the reducing elements of iron and sulfur in the surface rocks sequestered the oxygen of the water molecules. These reactions set hydrogen free as a gas, the light atoms escaping into space. The solar ultraviolet at the edge of the atmosphere may also have split some water vapor into hydrogen and oxygen. Either way, hydrogen, and hence water, was lost forever and the planet made more oxidized. Venus now, with its furnace heat and brimstone-laden air, is a model for Hell. By comparison, the Earth is Heaven for the life it bears. How have we kept our oceans? It seems likely that the presence of life has done it. Robert Garrels tells me that his calculations suggest that, but for life, the Earth could have dried out in about 1.5 eons, midway through the Archean. There are several ways of retaining hydrogen on a planet. One is to add oxygen to the atmosphere or environment so that it captures hydrogen to form water. Life, in the act of photosynthesis, splits carbon dioxide into carbon and oxygen. If some of the carbon is buried in the crustal rocks, there remains a net increment of oxygen. For every atom of carbon buried, two atoms of oxygen are left behind. Each atom of carbon buried, therefore, is in effect four atoms of hydrogen or two molecules of water saved. Then there are the reactions at the ocean floor between sea water and the ferrous iron in basalt rock. The free hydrogen that these produce would be food for the bacterial species who could gain energy by using it to make methane, hydrogen sulfide, and other compounds less volatile than hydrogen. Methane, decomposed in the atmosphere by ultraviolet, could stratify the atmosphere and slow the rate of mixing of gases from the lower atmosphere, which would also hinder the escape of hydrogen to space. In these and other, more subtle ways, the presence of life in the Archean saved our planet from a dusty death. Elso Barghoorn and Stanley Tyler first discovered the fossil bacteria that led to the recognition of the presence and the form of life in Archean times. I once visited Barghoorn's laboratory at Harvard University, and saw for myself the exquisite technical skills he used to cut, with diamond saws, the thin transparent slices of flinty rock. In this way, he and Tyler found the microfossils of bacteria in the ancient Gunflint rocks of the Great Lakes region of North America. But all these ancient fossils are from wet places, and we still do not know if there was life on the dry land. I find it hard to believe that a life form as enterprising as bacteria would have left unused the land surfaces. At this point, I should like to tidy away what I believe to be a persistent false assumption about those early times. We are using a new theory to view the scene; it helps to have the few genuine pieces of evidence displayed on a clean sheet. The false image, that lingers like a mirage, is the shibboleth, "Earth's fragile shield." In a way, the atmospheric scientists L. V. Berkner and L. C. Marshall started it. Some thirty years ago, they introduced their famous theory on the evolution of atmospheric oxygen. Crucial to this was the assumption that there was a flux of lethal ultraviolet radiation before oxygen was present in the air and that this prevented life from colonizing the land surfaces. Indeed, it was further held that life before oxygen must have been obliged to exist deep in the sea at levels where the ultraviolet could not penetrate. It was only after oxygen appeared in the air that ozone could form and act as a shield to prevent the ultraviolet from reaching the surface. Once this happened, the way was open for an abundance of life to colonize the land and for the growth of oxygen concentration by increased photosynthesis to its present level of 21 percent. Some details of their theory we now suspect are wrong, such as that oxygen was at times more abundant than it is now. But this is no discredit; the information needed to test their theories was not then available. We owe an immense debt to Berkner and Marshall for the stimulating effect their ideas had on the development of the Earth sciences. Like Vernadsky and Hutchinson before them, they were scientists who presented a world model in which life had a part to play and was not just a spectator obliged to adapt to the climatic and chemical whims of a purely physical and chemical world. The scientific establishment accepted their ideas enthusiastically. Among those ideas was the minor postulate that the presence of a stratospheric ozone layer is an essential requirement for surface life. Almost every scientist now accepts it as if it were a proven fact of science. There could have been no ozone layer at the start of life and during the Archean; gases like hydrogen and methane were dominant in the atmospheric chemistry, and even if there had been some oxygen in the atmosphere it could not have been used to form ozone. (Ozone is produced when ultraviolet radiation in the stratosphere splits molecules of oxygen into two separate atoms, which then combine with other molecules of oxygen to form a three-atom variety of oxygen: O3.) The intensity of ultraviolet in the absence of ozone would have been 30 times higher than is now incident upon the Earth's surface. Such an irradiation, it is said, would have sterilized the land surfaces. The more committed believers in the potency of ultraviolet hold that 10 to 30 meters of ocean water are needed to filter out the deadly radiation. Life, they say, could not have existed in shallower depths of the sea, let alone on the surface. Much more probably, "Earth's fragile shield" is a myth. The ozone layer certainly exists today, but it is a flight of fancy to believe that its presence is essential for life. My first job as a graduate was at the National Institute for Medical Research in London. My boss was the courteous and distinguished generalist, Robert Bourdillon. I was privileged to watch, and later participate in, the experiments that he and my colleague, Owen Lidwell, made as they tried to kill bacteria by exposing them to unfiltered ultraviolet radiation. Our practical objective was the prevention of cross infection in hospital wards and operating theatres. We were seeking a way to kill airborne bacteria and so prevent the spread of infection. Naked washed bacteria of some species, when suspended in the air as fine droplets, were easily destroyed by ultraviolet. It was impressive, though, how small a film of organic matter would almost entirely protect even these sensitive species. In the real world outside the laboratory, bacteria do not exist suspended in distilled water or a saline solution. In their normal habitats, bacteria are clothed in mucus secretions or the organic and mineral constituents of their environment. They do not live naked anymore than we do. Many practical trials were made before it was realised that ultraviolet radiation is not an effective method of eliminating from the hospital environment the tender fragile pathogens. It takes almost no clothing to stop ultraviolet radiation. [1] The memory of these experiments left me disinclined to accept that the much weaker irradiation of the land surfaces in the Archean by natural ultraviolet could have prevented their colonization. The organisms then around were used to living outdoors in the sunlight and had millions of years in which to adapt themselves or the Earth. It is also wrong to assume that ozone alone among atmospheric gases can filter out ultraviolet light. Many other compounds absorb and remove shortwave ultraviolet radiation. The most probable candidates in the Archean would be the smog-like products from the decomposition of methane or hydrogen sulfide. In the ocean there are even more possibilities. The abundant ions of such transition elements as iron, manganese, and cobalt are intense absorbers of ultraviolet, as are the ions of nitrous acid and of many organic acids. But even if the full unfiltered solar ultraviolet shone on the surface, it still would not have much hindered life. Organisms are nothing if not opportunistic. They would probably have turned the hard ultraviolet light to use as a premium energy source. It is an insult to the versatility of biological systems to assume that a weakly penetrating radiation like solar ultraviolet could be an insurmountable obstacle to surface life. Even dark-skinned humans are almost immune to its effects; and it is used in the skin of us all for the opportunistic photobiochemical production of vitamin D. This belief that ultraviolet radiation is unconditionally lethal to life on Earth has sustained a distorted view of the Archean and of other periods in the evolution of Gaia. And it is a view still deeply entrenched in scientific thinking. I found it to be common among the scientists who sought life on Mars. I could not help wondering how they could think that there was life on the intensely irradiated surface of Mars and at the same time believe that the land beneath the thick and murky Archean atmosphere of Earth was sterile. How could they fit into their minds two such contrary ideas? I think that a more serious threat to the health of land colonies in those times would be the need for rain. Rainfall on the continental land masses of the present Earth is, to a considerable extent, a consequence of evapotranspiration: the pumping by trees and large plants of water from the soil to their leaves where it evaporates. The rising plumes of water vapor over forests act like invisible mountains and force the inflowing air from the oceans to rain out its burden of water. Even if bacterial life grew to form stromatolites, it is unlikely that these colonial structures that rose above the surface would be as efficient at rain making as trees. (Small though they are, however, bacteria do have tactics for rain making. Recently, scientists have found that bacteria of Pseudomonad type synthesize a macromolecule which can induce freezing in water droplets supercooled below O°C.) Although bulk water, as in a swimming pool or even in a glass, freezes when its temperature falls below O°C, droplets of water that have condensed inside a cloud may not freeze until the temperature falls to -40°C. This supercooling takes place in the absence of nuclei of solid particles onto which the first microscopic ice crystal can form and grow. Pure water is reluctant to freeze; it freezes in our refrigerators because, in bulk water, there is always at least one nucleus to start the process of nucleation. Some chemical substances, such as silver iodide, have crystals close enough in shape to mimic ice. If these are dusted on a supercooled cloud, they will start the freezing process and sometimes the fall of rain. The macromolecule that the pseudomonads synthesize can cause droplets cooled only to -2°C to freeze, and is far more efficient than silver iodide. (This has led to commercial interest in methods of rain making. Silver iodide crystals work after a fashion, and production of the efficient pseudomonads macromolecule is under way. But it is thought by some environmentalists to be socially undesirable; the stealing of rain that might otherwise have fallen on those who may have needed it more.) Pseudo monads have an ancient history, and maybe their ice-nucleation trick goes back to the Archean. If so, were they the rain makers that led the colonization of the land? A question that always arises at this point in speculation is: How did it happen? Surely the bacteria did not decide to make the ice-nucleating substance. At this point, serious-minded microbiologists grow anxious and fear the proximate occasion of teleological heresy. Fortunately, we can easily make a plausible model of the evolution of close coupling between a large-scale environmental effect and the local activity of microorganisms -- a model, moreover, free of any taint of purpose. It is probable that the regional and global physiological systems of Gaia have their origins in some local competition and negotiation between species. An early variant of the ice maker may have found that the freezing of dew at its growth site gave some advantage. It might have been destroying a competitor or predator by freezing, splitting the tough skin of a food organism, or producing mechanical fractures in rocks to release nutrients or increase the quantity of soil particles. Any of these effects, alone or in combination, would confer advantage on the ice maker and, more important, favor those that made the most or the best nucleator. Eventually, the best possible nucleator would be ubiquitous in its distribution. For purely local reasons, these bacteria would continue their freezing activity wherever it was to their advantage. It is not difficult to see that surface ecosystems carrying ice makers would be at an advantage under drought conditions compared with those unable to produce the nucleating agent. The soil dust stirred by the wind or lifted by whirlwinds could induce droplet freezing in the clouds and then rainfall. The connection between the freezing of cloud droplets and the subsequent fall of rain is well understood. A great amount of heat is released when water freezes; in other words, freezing half of the water in a drop supercooled to -40°C releases enough heat to raise the temperature of the mixture of water and ice by 40° to the freezing point. If a large proportion of supercooled droplets in a cloud freeze, the latent heat released warms the cloud and causes it to rise. More water vapor condenses and freezes so that ice and snow falling though the cloud gather water and weight, and fall as rain. Any product of living organisms that nucleates supercooled cloud droplets will therefore encourage rain. More important in climate regulation than the nucleation of supercooled water droplets is the nucleation of supersaturated water vapor. The air above the open ocean is often supersaturated with water vapor. But no clouds or moist droplets can form until fine particles, the cloud condensation nuclei, appear. The climatologist Robert Charlson has argued that the emissions of sulfur compounds by the biota now and in the recent past has played an important role in providing cloud condensation nuclei. But this requires the presence of atmospheric oxygen to oxidize the sulfur to sulfuric and methanesulfonic acids, the nucleating agents. This could not have happened in the Archean, but there may have been other molecular species that served in this way. The aerosol of sea salt from breaking waves has some capacity to nucleate clouds, but it is slight compared with that of the sulfur acid micro-droplets. Although rainfall is essential for growth on the land, it also poses problems because it washes away nutrients. (The poor productivity of the rain-washed uplands of the west coast of the British Isles is an example of this problem.) Today, rivers carry to the ocean elements that are used or required by marine life -- such as nitrogen, phosphorus, calcium, and silicon. But the rivers also carry the rarer elements -- sulfur, selenium, and iodine -- to the sea, and the land becomes depleted. This brings us to another large-scale geophysiological mechanism: the transfer of essential or nutritious elements from the ocean, where they are abundant, to the land, where they are scarce. The process requires marine life to synthesize specific chemical compounds that act as carriers of the elements through the air. The element sulfur, for example, is carried from the ocean to the land by dimethyl sulfide, a product of marine algae. In the Archean, the environment was either oxygen-free or there was an excess of reducing gases over oxygen. In such an atmosphere, the synthesis of dimethyl sulfide, which seems to take place only in oxic environments, is unlikely. Compounds such as hydrogen sulfide and carbon disulfide, which are unstable in our present oxidizing air, could have served instead to carry the essential element sulfur, also in the Archean land life could have needed less. Hydrogen sulfide is ubiquitous in the anoxic zones and reacts with many metals -- such as lead, silver, and mercury -- that might otherwise accumulate to toxic levels. The result is water-insoluble sulfides that settle as solids. The geochemist Wolfgang Krumbein has shown that the ore beds of these elements exposed on or near the surface today are the waste tips of some past anoxic ecosystem. Anaerobic organisms that converted the potentially toxic elements, mercury and lead, to their volatile methyl derivatives grew successfully and provided the ecosystem with a mechanism to remove toxic waste. The anoxic zones are continuously perfused by a flow of methane gas that would serve to carry these volatile materials away from the region. Some of this methylating activity is beneficial on a regional or even global scale. The production of dimethyl selenium serves in a subtle way, first discovered by the atmospheric chemist F. S. Rowland, to offset the toxicity of dimethyl mercury. It also acts to recirculate the essential element selenium through the global environment. The rate of carbon burial during the Archean was not significantly different from today. As we saw earlier, the carbon present in the earliest sedimentary rocks shows a subtle difference in the proportion of its isotopes from that of lunar rocks that have never been exposed to life; this difference is evidence for the presence of photosynthesizers. The geologist Euan Nisbet tells me that there are Archean, carbon-rich shale deposits in southern Africa. They are like the coal measures put down by the forest trees of the Carboniferous period, eons later. These carbon deposits are all that remains of the dead bodies of microorganisms that once grew in the Archean. Volcanoes then, as now, vented carbon dioxide. Archean photosynthetic and other bacteria used this carbon dioxide to make the organic compounds of their cells; these organisms also may have facilitated the reaction of carbon dioxide with calcium and other divalent ions dissolved in the sea and on the land surfaces. These two reactions were the sinks for carbon dioxide and kept a steady level in the atmosphere. This is part of the climate regulating system illustrated in figure 4.2. In addition to these climatic consequences, the Archean ecosystems would have buried a small but constant proportion of their carbon turnover, which would have led to the steady addition of oxygen. This, however, would have been used up in oxidizing the reducing compounds of the surface and ocean environments, and that emitted by volcanoes. It was somewhat like one of those chemistry experiments in high school, where you progressively add an oxidizing solution to a reducing solution until an indicator suddenly changes color to mark the equally sudden change from reducing to oxidizing at the end of the titration. The burial of a small proportion of the carbon and sulfur, cycled by once-living bacteria, titrated the oxidizable material of the environment until the surplus was used up. Reducing material continued to be added to the ocean and the atmosphere, but the rate of its addition became less than that of carbon burial. Free oxygen gas began to appear in the air at levels more than sufficient to overcome the reducing tendency of methane, and marked the end of the epoch. It seems likely that the end of the period when methane dominated the chemistry of the atmosphere was abrupt. But it would be wrong to envisage a sudden change from a wholly oxygen-free world to one where oxygen was present free in the air. Much more probable is the gradual growth during the latter part of the Archean of oxic organisms at the surface of the Earth. These could have existed first at the surface, where the phototrophs basked in the sunlight and locally produced enough oxygen to support them. They would be a separate and encapsulated ecosystem surviving in an otherwise lethal system, rather as the anaerobes survive in the poisonous oxygen-rich world of today. In this oxic ecosystem there would be consumers living on organic products of the cyanobacteria, and also organisms able to exploit a slightly oxidizing medium and perform such tricks as denitrification (using nitrate and nitrite ions instead of oxygen, so that nitrogen escaped to the air as gaseous nitrogen and nitrous oxide). Gradually, as the oxygen-scavenging compounds of the sea were used up, the oxygen released by the phototrophs would no longer be absorbed. Then the ratio of the methane to oxygen flux to the atmosphere would shift towards an oxygen excess. The oxic ecosystems would spread and, just before free oxygen increased in abundance to become the dominant oxidizer, would probably have covered most of the oceans. The changeover was not so much a genocide as a domination. Even stranger scenarios are likely if the surface communities generated nitrous oxide before oxygen itself appeared. This gas is stable in the troposphere and might have allowed methane to persist longer; it is also somewhat of a greenhouse gas and might have compensated for the decline in methane. It is made by bacteria now, and it is likely that there were bacteria making it then. In geophysiology, the Archean boundary coincided with the great punctuation marked by oxygen's free presence in the air. However, for the bacteria of the Archean the era never ended. They live on wherever the environment is free of oxygen. They run the vital and extensive ecosystems of the anoxic zones beneath the sea floor, in the wetlands and marshes, and in the guts of nearly all consumers including ourselves. In a strict geological sense, the period ended 2.5 eons ago, and oxygen may have come later. The appearance of oxygen in the air and on the surface of the oceans did not eliminate the anoxic ecosystems; it merely segregated them in the stagnant waters and sediments. As a consequence, the rocks that formed from these sediments may have failed to record the presence of free oxygen in the air. That, then, is an account of a few aspects of the Archean seen through Gaia theory. It was a period when the Earth's operating system was populated wholly by bacteria. It was a long period, when the living constituents of Gaia could be truly considered as a single tissue. Bacteria are both mobile and motile, and could have moved around the world carried by winds and ocean currents. They can also readily exchange information, as messages encoded on low-molecular-weight chains of nucleic acids called plasmids. All life on Earth was then linked by a slow but precise communication network. Marshall McLuhan's vision of the "global village," with humans tied in a chattering network of telecommunication, is a re-enactment of this Archean device. _______________ Notes: 1. Those still skeptical might be persuaded by the reports of these experiments in the Medical Research Council's special report number 262, entitled "Studies in Air Hygiene" and published in 1948.
|
|||||||||